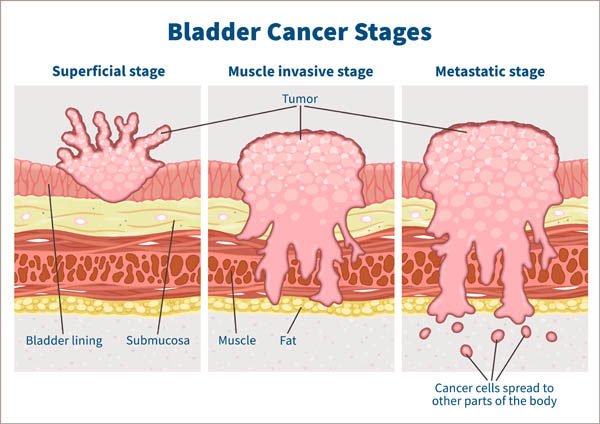
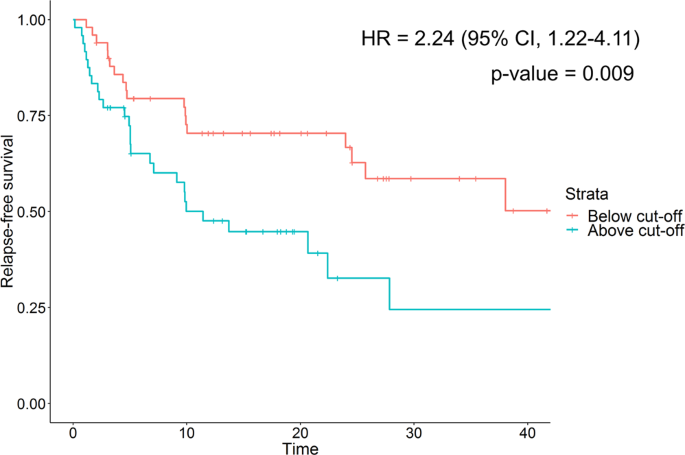
Multiple recurrences and risk of disease progression in patients with primary low-grade (TaG1) non–muscle-invasive bladder cancer and with low and intermediate EORTC-risk score | PLOS ONE
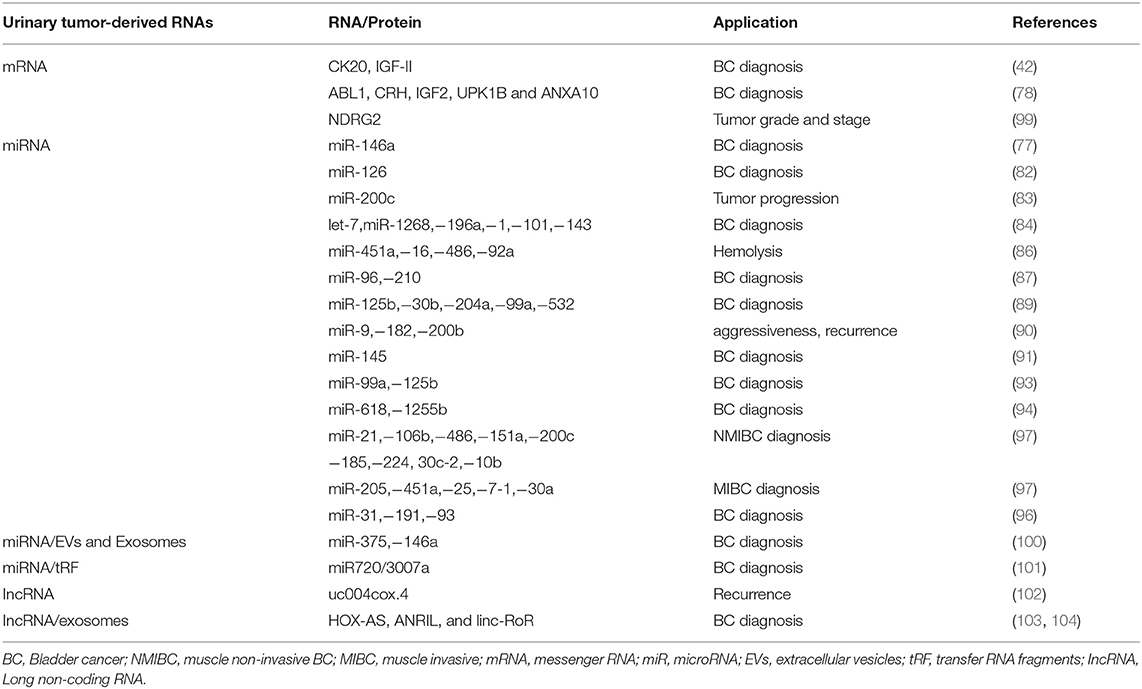
Cancers | Free Full-Text | Noninvasive Urine-Based Tests to Diagnose or Detect Recurrence of Bladder Cancer

Urinary TERT promoter mutations are detectable up to 10 years prior to clinical diagnosis of bladder cancer: Evidence from the Golestan Cohort Study - eBioMedicine

Artificial intelligence: A promising frontier in bladder cancer diagnosis and outcome prediction - ScienceDirect

Unmet Clinical Needs and Future Perspectives in Non–muscle-invasive Bladder Cancer - European Urology Focus

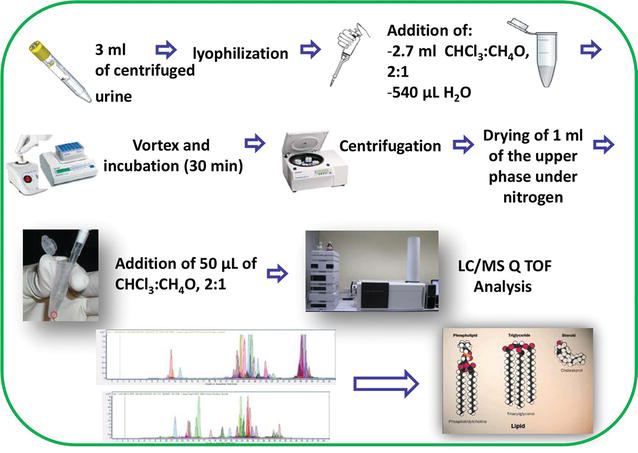
Urinary biomarkers to mitigate diagnostic delay in bladder cancer during the COVID-19 era | Nature Reviews Urology

Recurrent ZNF83-E293V Mutation Promotes Bladder Cancer Progression through the NF-κB Pathway via Transcriptional Dysregulation of S100A8: Molecular Therapy
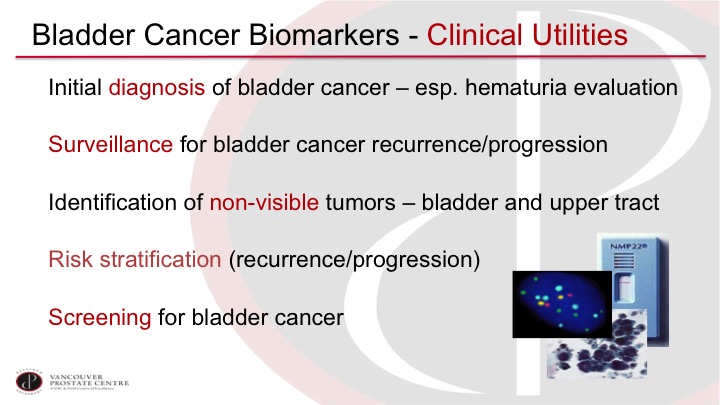
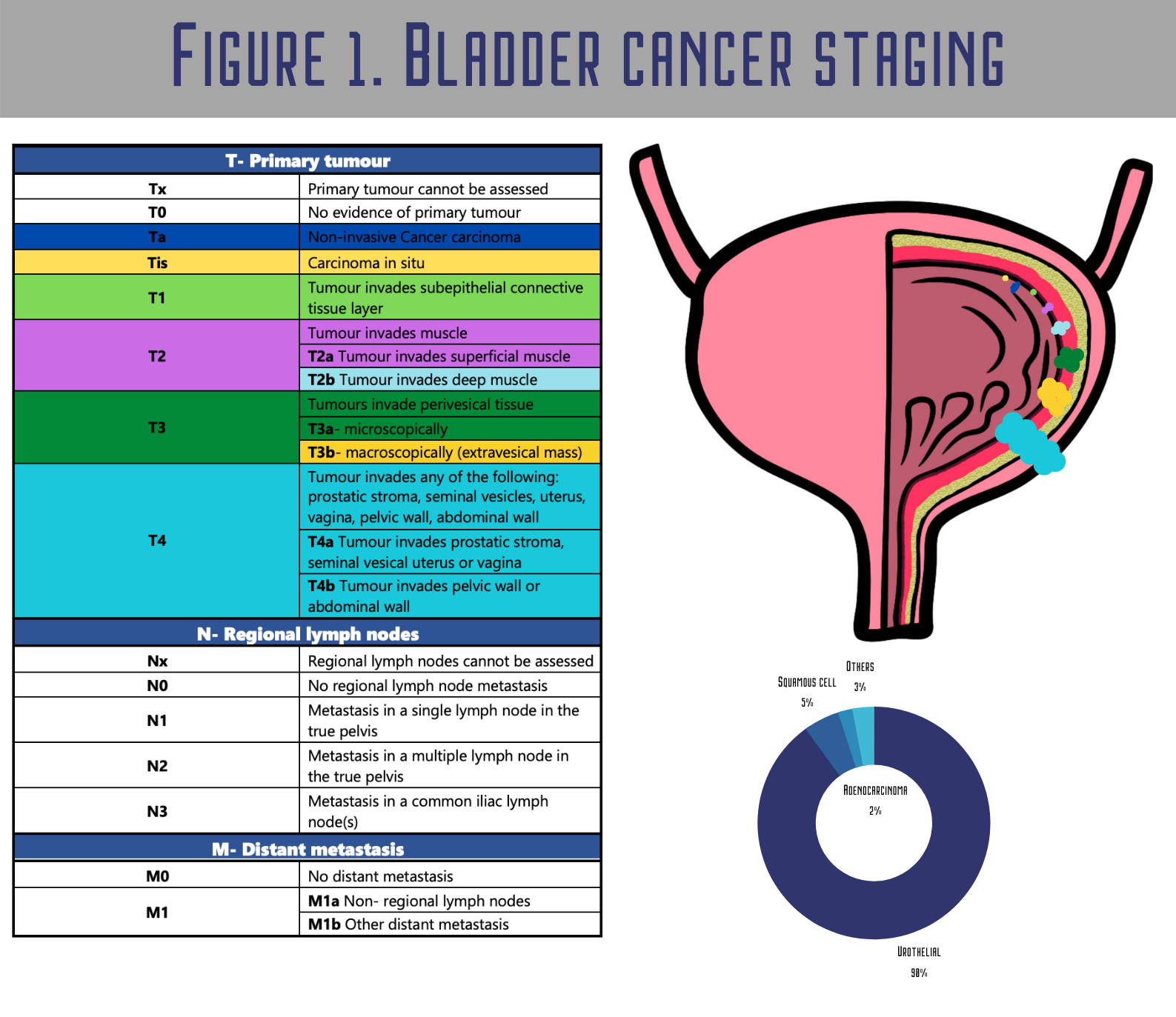
This policy documents the coverage determination for Urinary Tumor Markers for Bladder Cancer. The diagnosis of bladder cancer i

JCI - Urine DNA methylation assay enables early detection and recurrence monitoring for bladder cancer

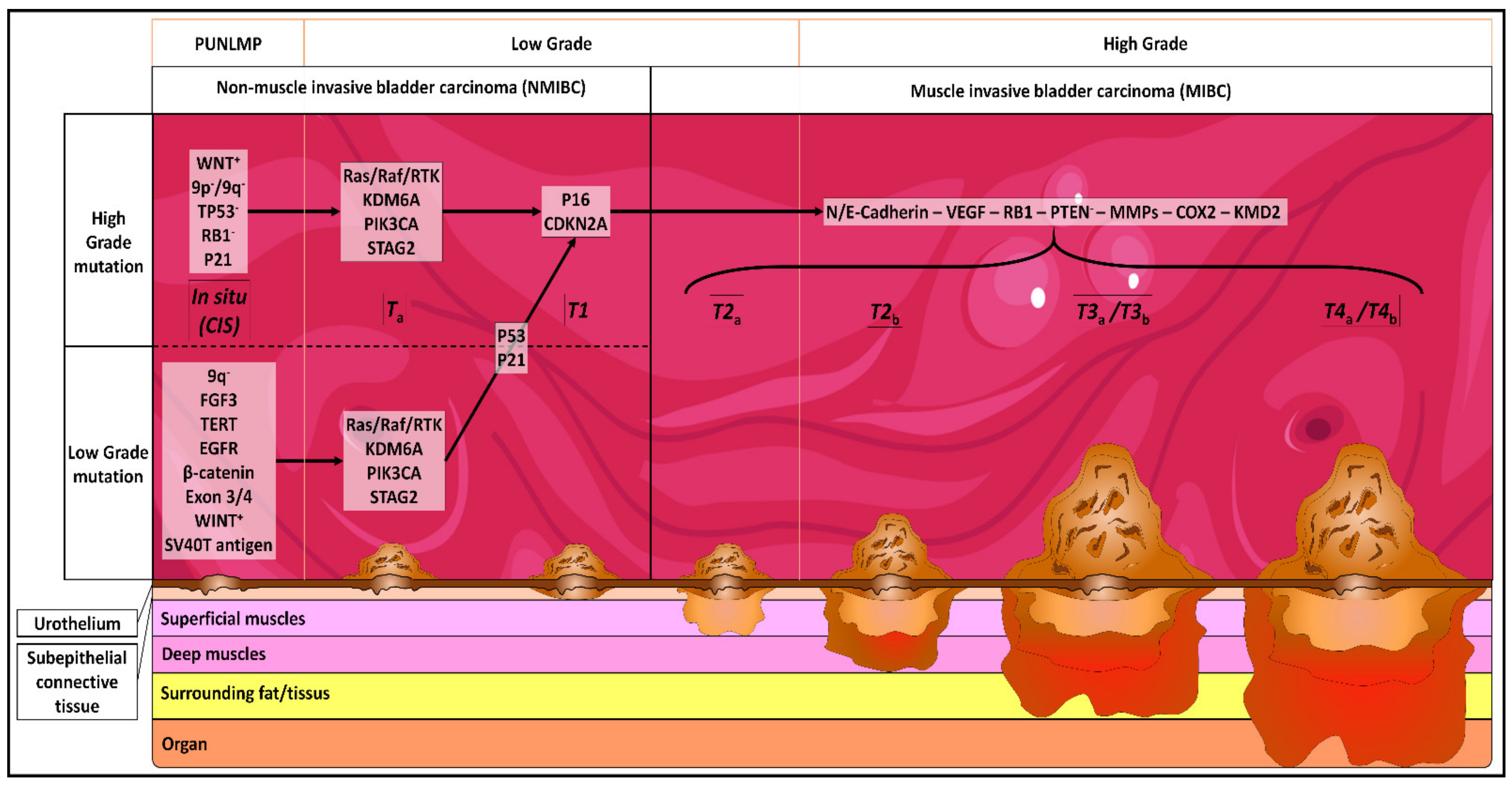
Stage-stratified molecular profiling of non-muscle-invasive bladder cancer enhances biological, clinical, and therapeutic insight - ScienceDirect

Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study - The Lancet Oncology